Document Management
Upload Document
Create Document From Template
Before adding Quality Management Documents
Before adding qms documents, you must already have following masters ready.
Once you have these ready, you can start adding your existing documents to the quality management system.Adding QMS Document Categories
Adding Document categories, is same as adding any masters. You can add document categories one-by-one or use Bulk Add method.
Click on at the top, which will take you to the Document Category section. Click on button to add categories one-by-one or use Add Bulk button for add bulk.
While Bulk Add, use CSV format where each each new category will be added as new line <Enter> and Category Short Name and Category Name will be seperated by COMMA. e.g:
Short Name, Name<Enter>
M,Manual
F,Format
P,Procedure
Proceed with document creation once all these masters are ready.
Best Practices
Make sure that all the Standards & Clauses are added properly
Make sure that all the Document Categories are added and linked with correct Standards
If you already have existing documents, keep them handy in a folder, so you can upload them easily
Avoid spaces, special characters in file names
Adding your First QMS Document
Click on icon at the top menu to goto QC Document index page.
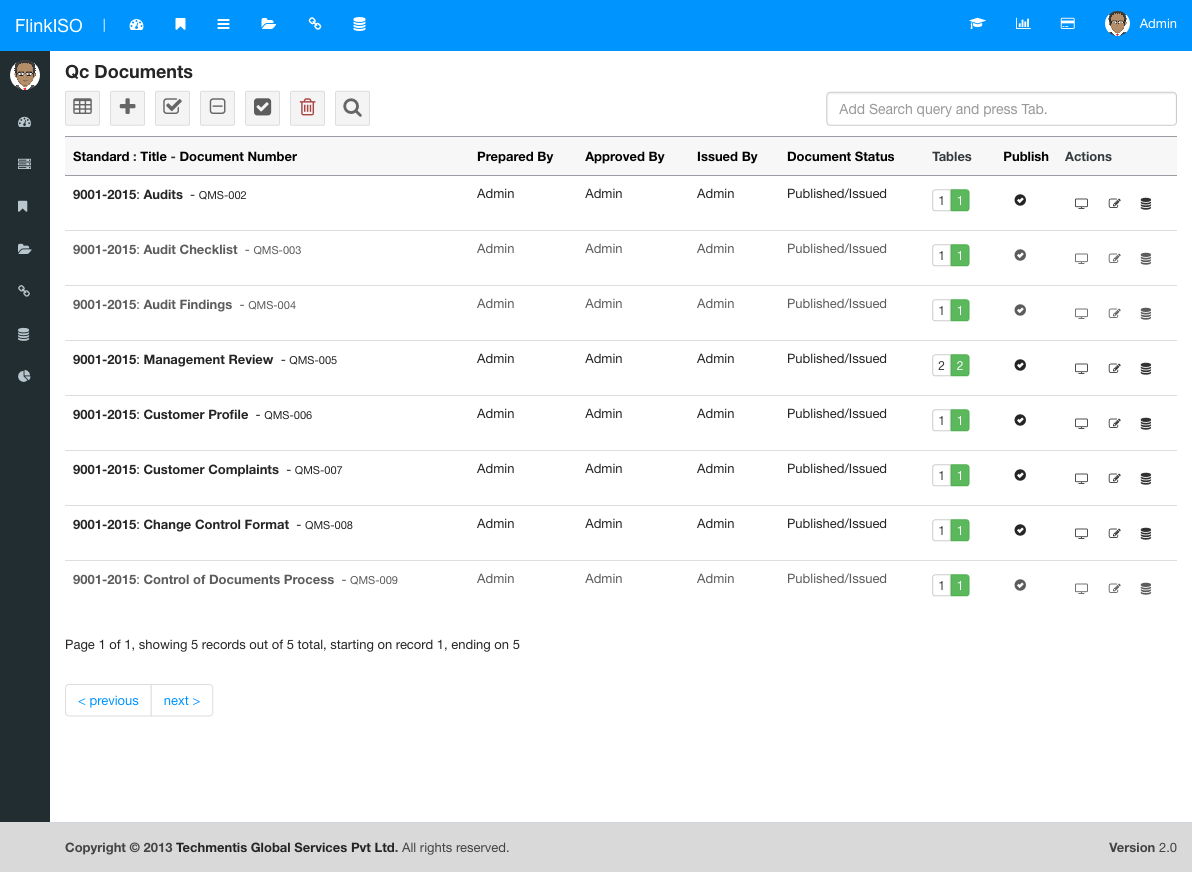
Click on icon at the top menu to goto Add New QC Document form.
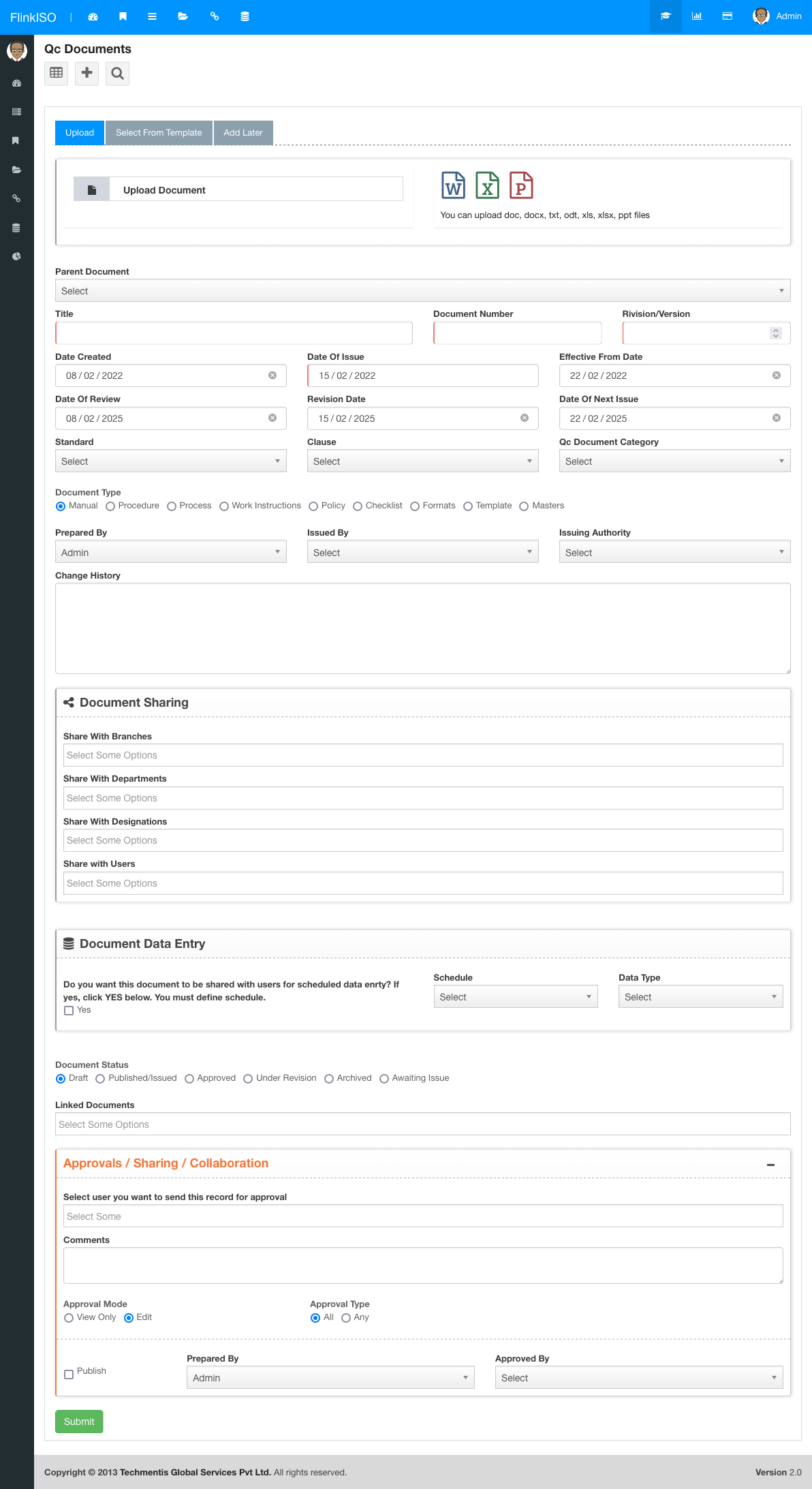
| Allowed File Types | doc, docx, txt, odt, xls, xlsx, csv, ppt |
| Allowed File Size | Max 25MB |
| Parent Document | If the document is derieved from any parent document |
| Document Number | Number as per your document |
| Document Version Number | Version Number as per your document |
| Date Created | Document Creation Date |
| Date Of Issue | Wnen document is issued |
| Effective From Date | Date from which document will be adopted |
| Date Of Review | +3 Years from Date Created |
| Revision Date | +7 days from Review Date |
| Date Of Next Issue | +7 days from Revision Date |
Note:
If the document you are adding, already has a change history, add it here.
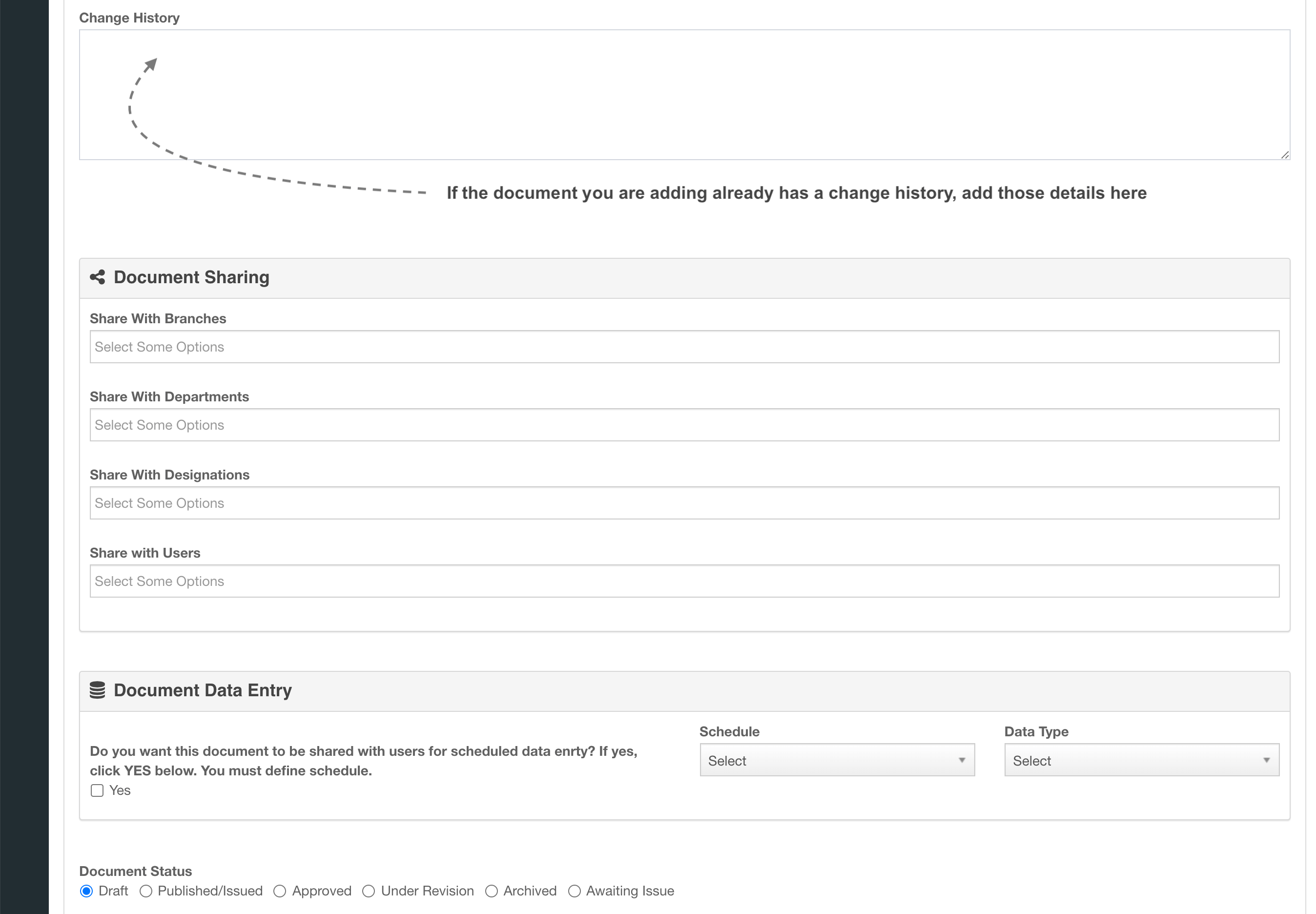
Document Sharing / Distribution
You can share a document while creating it or uploading it. You can share the document Branch-wise, Department-wise, Designation-wise or User-wise
Note:
When you are adding the document, share it only with Approver & Issuer and/or with the member who is co-authering the document with you
Once the document is approved & issued, share the document with rest of the members, while you are Publishing the document
You can change the document sharing from Edit page
Note:
When you share the document with the member/s, they will be able to see these shared documents on their dashboard. These users can Open & Edit these shared documents in draft mode along-side with the creator or other members (see Real Time Document Collaboration section below).
FlinkISO™ uses ONLYOFFICE™'s colloberation module for this purpose.
Once the document is ready, you can use FlinkISO™ Approval Process to send the document to Approver & Then Approver can forward this document to Issuer for final issue. While issuing the document, mark the document as Published/Issued and add rest of the members who can access this document.
Note:
Real Time Document Collaboration & Auto Version Control
Co-author documents with your colleagues in run-time. Upload and save the document in Draft mode, then open the document from Edit page and share with whom you would like to share to the document. Add their names to Sharing Panel. Turn on "Co-Editing Mode" from the Edior and Click Submit.
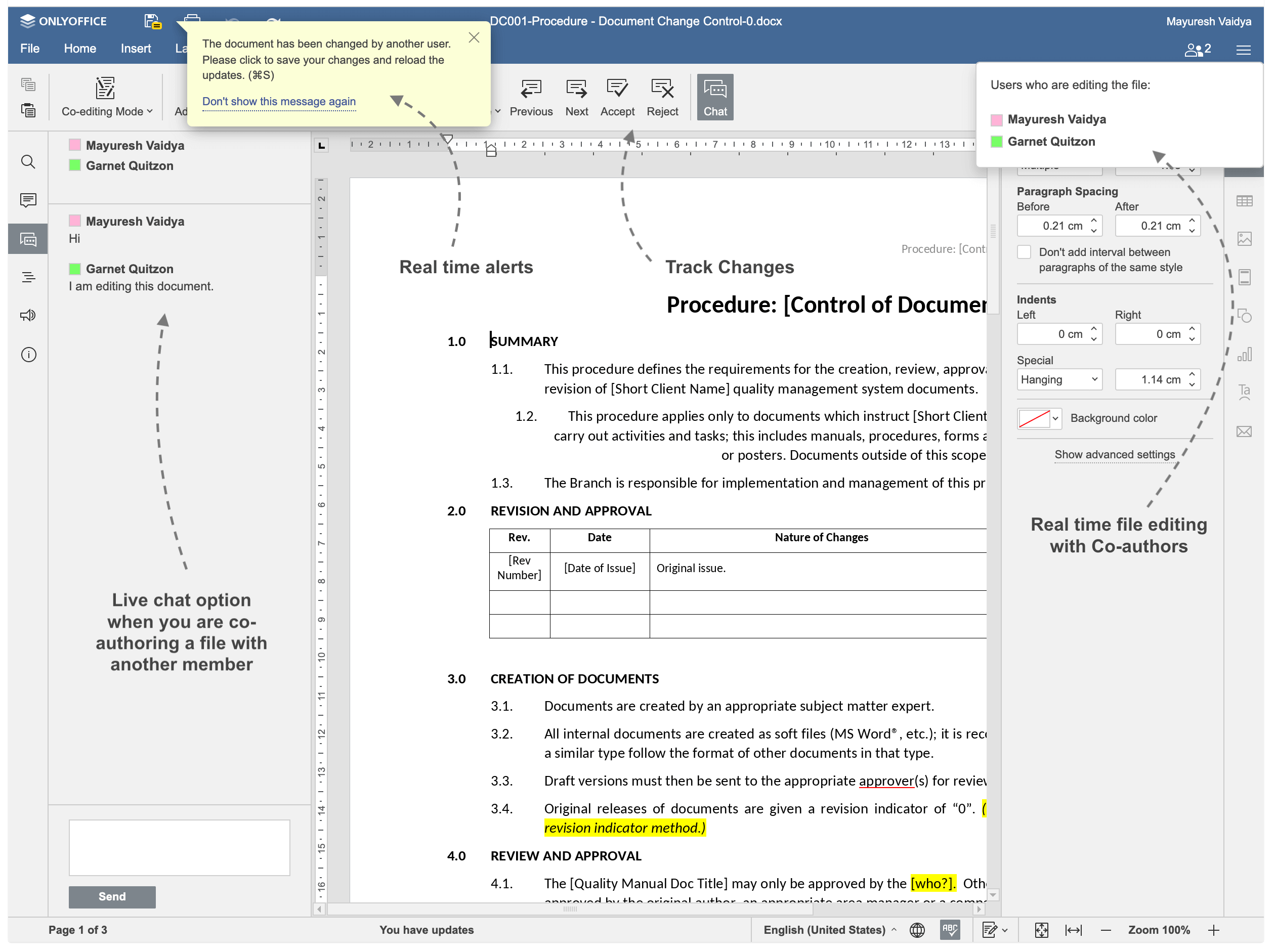 Note: Document & its content inside editor is only for demo purpose only. We do not provide/sell any manuals,formats along with the application.
Note: Document & its content inside editor is only for demo purpose only. We do not provide/sell any manuals,formats along with the application.
More Features
ONLYOFFICE Doc Editors
With ONLYOFFICE™ Editors, you have complete control on your documents without loosing any formatting unlike online web-based HTML editors.
Document Version Control
Each and Every QMS document created/ uploaded in the QMS is Version Controlled and Access Controlled and follows all the Document Management best practices.
Security
Add password to secure your PDFs while you download them. System records every downloads with issue number & timestamps along with user details.
Validation
HTML forms created are automatically version controlled, ready with required validations as well as design/ layout you choose while creating these HTML forms.
Access Control
Each of these forms are either linked with the QMS document and by default inherit access restrictions based on document access.
Approvals
Every form created, by default follows system's approval system. Users with limited roles, cannot publish any record in the system unless that record/ document is approved by HoDs or Administrators.
Lossless Migration
With ONLYOFFICE™ Editors, you can preserve all your formulas and sheets in your spreadsheets.
Upload / Create
Simply upload the existing documents/ spreadsheets/ presentations to create the document or spreadsheet.
Scheduled Data Entry
You can easily create spreadsheets, documents, formats etc and share with users within the system for scheduled data entry, eliminating efforts of printing and distributing these documents manually.
Centralized Data
Users can open these shared documents from within the system, add their data and save them onto the system without any need to download/ upload. All data is stored in a singled location and can be accessed secuerly.
Drag and Drop
To store this data in SQL format instead of Document format, you are free to build HTML forms within the system by using our Drag-and-Drop feature.
Digital Signature
Digitally sign all your QMS document while downloading them in PDF format. You can draw your signature or upload existing signature to the system.
